
Regulatory Toxicity Testing: A course review
Hello everyone and welcome back to my blog and to this new year 2021! I hope you all had an amazing and relaxing Christmas break and that despite the circumstances you enjoyed a lot! This time I will review the last course of the Toxicology programme: Regulatory toxicity testing. During this blog, I will discuss and explain in detail how this course is structured and carried out. In addition, I will highlight some of the most relevant aspects and the reason why this course was so different from other courses. Finally, this will be my last course review blog, so I hope you enjoy it!
Course structure
Regulatory Toxicity Testing is the very last course of the Toxicology programme. It lasts approximately 6 weeks and comprehends a total of 10 credits. In comparison with other Toxicology courses, this one is a very different one. But why is it so different? Well, this is because the course’s main structure relies on a final pre-clinical group report in which we all have to collaborate as a big team. However, before collaborating as a whole group we are divided in four smaller groups. As a consequence, each group is assigned a specific topic to work with. During this process, each group writes methodology, works and analyzes raw data. Subsequently, before putting the report’s parts altogether, each group assigns two communicators. The communicators’ task is to make sure that everything in the final report is harmonized and correct (I will explain the group report in detail later on).
Also, during this course we had very few lectures from different RISE (Research Institutes of Sweden) experts. Such lectures aimed at giving us an overview of all the topics the group report would contain. Besides the lectures, we also had tutor and group meetings to discus our report’s part progress and doubts. To sum everything up, Regulatory Toxicity Testing is a course in which we learn and simulate how a real pre-clinical toxicity report is done and structured.
Topics
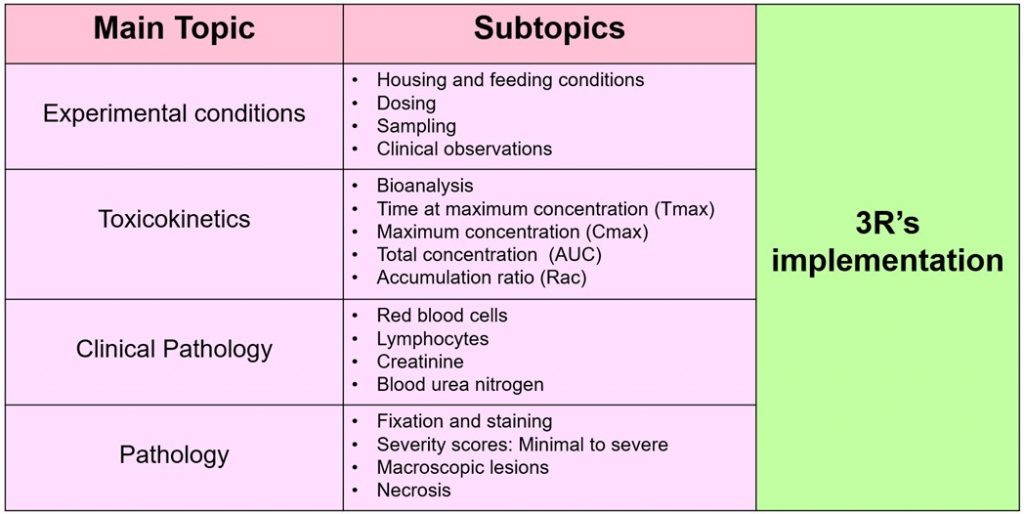
Due to the course’s nature, there were only four main topics: Experimental conditions, Toxicokinetics, Clinical Pathology and Pathology. Of course, each of the topics had its own specific subtopics which I will explain in the next table:
Group report
Now that I have discussed on the course structure and the main topics involved, is time to explain in detail what the group repot consists of.
The report is basically the simulation of a real pre-clinical drug. For this, we actually get raw data from previous studies. However, because of confidentiality of the novel drug involved we do not get any names or brands. Indeed, the aim is to learn and get familiar with the process of testing and reporting toxicity in vivo of a drug. Usually, the drug has already been tested in vitro or has been used in veterinary medicine.

As I mentioned before, the report is built up from all the four groups’ part and the communicators work as editors. This implies that during the first weeks, all members of all groups will work on their individual parts. First, based on the given SOPs, each group will write the corresponding method section and then present it. Then, we process and analyze raw data and do statistical analysis when possible. Later, we work on discussion and conclusion for each of the sections. Finally, the editors put the report altogether and make sure that everything is complete and in order. Before submitting a final version, the whole group gives an oral presentation. In this presentation RISE experts are present and give feedback.
Evaluation
Regarding this course’s evaluation, it is basically divided as it follows: Toxicity testing, planning, performance and reporting (report) 6 credits and integration of toxicity testing (exam) 4 credits. As how it usually happens with the reports, there is only pass (G) or fail (U). In contrast, the exam is what will determine your final degree. In this one you can get pass with distinction (VG), pass or fail. The exam consists of around 32 open questions that involve all topics from lectures and the report results. Obviously to pass the course satisfactorily you must have a pass in both of the sections.
I hope you enjoyed this last blog and if you have questions on this course, do not hesitate and contact me!
Aline Colonnello
gloria.aline.colonnello.montero@stud.ki.se
Aline Colonnello - Toxicology
My name is Aline Colonnello Montero, I am twenty five years old and I come from the wonderful but busy Mexico City. I consider myself to be a perseverant person who works hard to meet all my goals and ambitions. I have a bachelor’s degree in Biology and I currently study the Master’s programme in Toxicology at Karolinska Institutet. My job as part of the digital ambassadors’ team consists on writing blogs

1 comments